Mohammed Jazara's Science Notebook
Problem:
What are some signs that a chemical reaction has taken place?
Materials:
-
4 - 100mL Beakers
-
Tea Candles Birthday candles
-
Sugar
-
Tongs
-
Clay
-
Matches
-
Sodium carbonate
-
Graduated cylinder, 10mL
-
Aluminum foil 10 cm square
-
Dilute hydrochloric acid
-
Copper sulfate solution
-
Sodium carbonate solution
Procedure:
Download data table from Moodle.
Part 1:
-
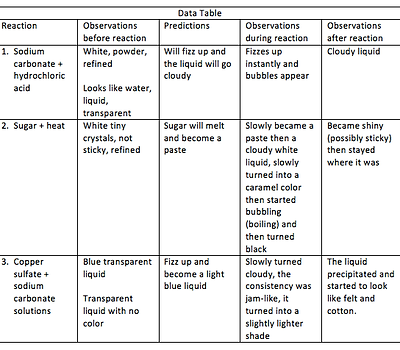
Put a pea-sized pile of sodium carbonate into a 100mL beaker. Record in the data table the appearance of the sodium carbonate.
-
Observe a dropper containing hydrochloric acid. Record the appearance of the acid.
-
Make a prediction about how you think the acid and the sodium carbonate will react.
-
Add about 10 drops of hydrochloric acid to the sodium carbonate. Swirl to mix the contents of the beaker. Record your observations.
Part 2:
-
Fold up the sides of the aluminum oil square to make a small tray.
-
Use a spatula to place a pea-sized pile of sugar into the tray.
-
Carefully describe the appearance of the sugar in your data table.
-
Predict what you think will happen if you heat the sugar. Record your prediction.
-
Take the tea candle and your aluminum square with the sugar outside. Carefully light the candle with a match only after being instructed to do so by your teacher.
-
Use tongs to hold the aluminum tray. Heat the sugar slowly by moving the tray gently back and forth over the flame. Make observations while the sugar is heating.
-
When you think there is no longer a chemical reaction occurring, blow out the candle.
-
Allow the tray to cool for a few seconds and set it down. Record your observations of the material left in the tray.
Part 3:
-
Put about 2 mL of copper sulfate solution in one beaker using a pipette. Caution: Copper sulfate is poisonous and can stain your skin and clothes. Do not touch it or get in in your mouth. Using a 10mL graduated cylinder, measure 2mL of sodium carbonate solution in another beaker. Record the appearance of both liquids.
-
Write a prediction of what you think will happen when the two solutions are mixed.
-
Combine the two solutions and record your observations.
-
Wash your hands.
Data:
Analysis:
Which of your predictions were accurate?
Our 1st and 2nd predictions: Sodium carbonate + Hydrochloric acid.
What was the biggest surprise?
The last reaction: Copper sulphate + Sodium carbonate solutions. The end result was incredibly weird and surprising.
How did you know when the reaction in Part 1 was over?
When nothing was still going on, everything became sort of stable and stopped reacting.
What was the evidence of a chemical reaction in Part 1?
A change in it's appearance occured, the substance sodium carbonate changed from solid to liquid when combined and the substance also turned cloudy.
What was the evidence of a chemical reaction in Part 2?
The sugar's colour changed, first it was white, then caramel color then dark brown and later black. The state of matter changed from a solid to a liquid then a solid again.
Was the reaction in Part 2 endothermic or exothermic? Explain.
Exothermic because the sugar was giving off heat from the fire. If it were hydrothermic it would be the opposite.
Was the product of the reaction in Part 3 a solid, a liquid, or a gas?
It became a solid after the reaction.
Conclusion:
The signs that a chemical reaction has taken place can either be a change in colour, smell, consistency, state of matter or a sound can take place. It's often obvious that a reaction took place.
Where is the Evidence?