Mohammed Jazara's Science Notebook
Physical characteristics of carbon-
-
Soft
-
Dull
-
Grey or black
-
Non metal
-
Can scratch with a fingernail
What is carbon usually used for?
Because of its fitting properties, it’s usually used as an additive to methane, gasoline and kerosene. Another form of carbon is mixed with clay to make lead in pencils. Charcoal is also used for artwork, another way people use carbon is to filter or remove poisons/gases from the digestive system. It is also known as diamond and is worn as jewelry.
Physical characteristics of oxygen-
-
Colorless
-
Odorless
-
Tasteless
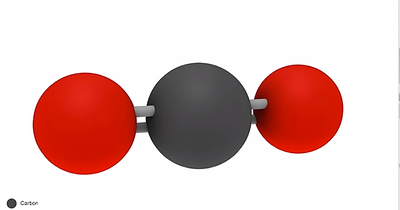
Characteristics after the reaction-
-
Colorless apart from when it’s a solid (dry ice), it then has a white color
-
Has a sharp acidic odor at high concentration
-
Odorless at low concentrations
-
Tasteless
-
Non-flammable
-
Heavier than air
-
Soluble in water
-
Slightly acidic
-
Harmful in large amounts (greenhouse gases)
-
Covalent molecule
-
Boiling point is -78.5 degrees Celsius
-
Melting point is -55.6 degrees Celsius
-
Released when burning fossil fuels
How many electrons does co2 have?
Carbon has 6 protons; Oxygen has 8 protons (two atoms though since it’s carbon Dioxide). The equation would be: 6+(8)2= 22. It has 22 protons therefore it has 22 electrons.
Elements carbon and oxygen